The Baking Soda and Vinegar Experiment: The Ultimate Guide
Ever witnessed a volcanic eruption in your kitchen? The classic baking soda and vinegar experiment is a staple of science fairs and a captivating activity for all ages. This guide dives deep into the science behind this fizzing phenomenon, providing a comprehensive understanding of the reaction, its applications, and how to conduct the experiment safely and effectively. We’ll explore everything you need to know to make this experiment a resounding success.
What is the Baking Soda and Vinegar Reaction?
The magic behind the bubbling eruption lies in a simple acid-base reaction. Baking soda (sodium bicarbonate – NaHCO₃) is a base, and vinegar (typically acetic acid – CH₃COOH) is an acid. When these two substances are mixed, they undergo a chemical reaction that produces carbon dioxide (CO₂), water (H₂O), and a salt (sodium acetate – CH₃COONa).
The Chemistry Unveiled: Breaking Down the Reaction
Let’s break down the chemical process in more detail:
- Reactants: Baking soda (NaHCO₃) and vinegar (CH₃COOH) are the starting materials.
- Reaction: The acetic acid in vinegar reacts with the sodium bicarbonate in baking soda.
- Products: This reaction produces:
- Carbon Dioxide (CO₂): This gas is what causes the fizzing and bubbling. It’s the same gas that makes soda fizzy!
- Water (H₂O): A common byproduct of many chemical reactions.
- Sodium Acetate (CH₃COONa): A salt that dissolves in the water.
The overall chemical equation for the reaction is:
NaHCO₃ (s) + CH₃COOH (aq) → CH₃COONa (aq) + H₂O (l) + CO₂ (g)
(s) = solid, (aq) = aqueous (dissolved in water), (l) = liquid, (g) = gas
Conducting the Experiment: A Step-by-Step Guide
Ready to unleash the volcanic potential? Here’s how to perform the baking soda and vinegar experiment:
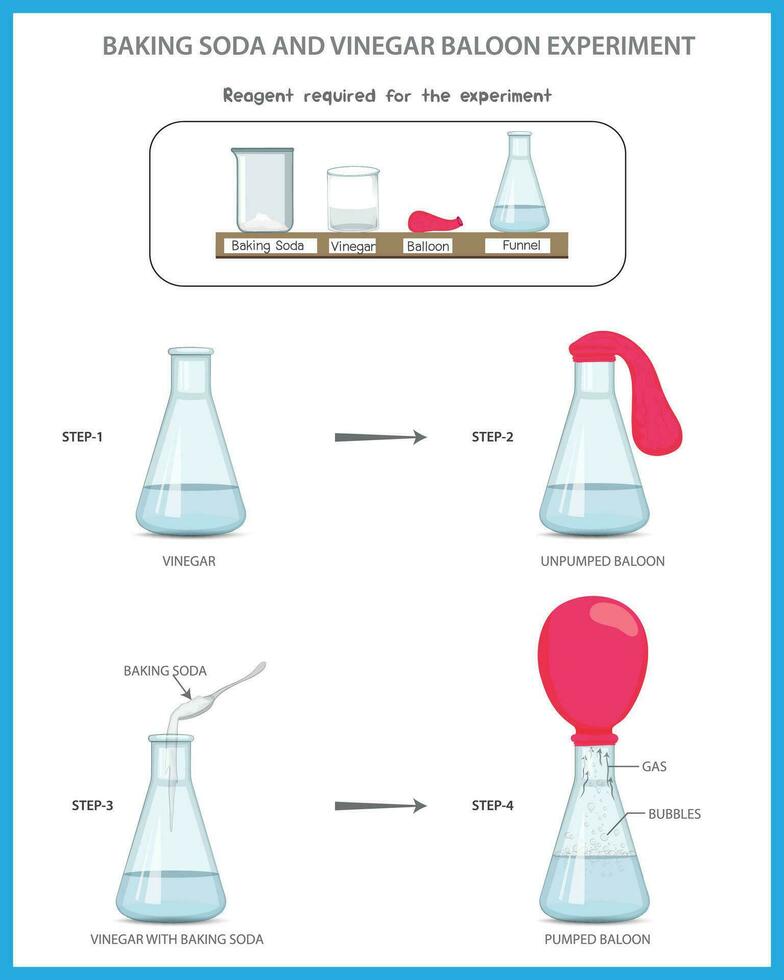
Materials You’ll Need:
- Baking soda (sodium bicarbonate)
- Vinegar (white distilled vinegar works best)
- A container (e.g., a plastic bottle, a glass jar, or a small volcano model)
- Optional: Food coloring, dish soap, glitter (for added visual appeal)
- A measuring cup and spoon
- A funnel (optional, for easier pouring)
Instructions:
- Prepare the Container: If you’re using a bottle, ensure it’s clean and empty. If you’re building a volcano, now’s the time to assemble it.
- Add Baking Soda: Using a measuring spoon, add 1-2 tablespoons of baking soda to your container.
- Add Optional Ingredients (if desired): Add a few drops of food coloring, a squirt of dish soap, or glitter for visual flair.
- Pour in the Vinegar: Slowly pour vinegar into the container. Observe the reaction!
- Observe and Enjoy: Watch the fizzing, bubbling eruption! The reaction will continue until one of the reactants is used up.
Enhancing the Experiment: Variations and Extensions
The basic experiment is just the beginning! Here are some ways to make it more engaging:
- Varying the Quantities: Experiment with different ratios of baking soda and vinegar to see how it affects the reaction’s intensity and duration.
- Temperature Effects: Try the experiment with warm and cold vinegar to see if temperature influences the reaction rate.
- Volcano Model: Build a volcano model using clay, cardboard, or other materials. This adds an element of fun and creativity.
- Measuring the CO₂: If you’re feeling adventurous, you can try to capture and measure the amount of carbon dioxide produced using balloons or other methods. This can lead to further scientific exploration.
Safety Precautions: Keeping it Safe
While the baking soda and vinegar experiment is generally safe, it’s important to take a few precautions:
- Adult Supervision: Young children should always be supervised by an adult.
- Eye Protection: Avoid getting vinegar in your eyes. If it does, flush thoroughly with water.
- Ventilation: Conduct the experiment in a well-ventilated area.
- Avoid Ingestion: Do not ingest the baking soda or vinegar.
- Clean Up: Wipe up any spills immediately.
Beyond the Fizz: Applications and Uses
The chemical reaction between baking soda and vinegar isn’t just fun; it also has practical applications:
- Cleaning: The reaction can be used to clean drains and remove stains.
- Baking: Baking soda is a leavening agent in baking, reacting with acidic ingredients like buttermilk or vinegar to produce carbon dioxide, which helps baked goods rise.
- Household Remedies: Vinegar can be used as a mild cleaning agent, and baking soda can neutralize odors.
- Educational Tool: The experiment is a fantastic way to introduce children to basic chemistry concepts.
Conclusion: Embracing the Science of Fizz
The baking soda and vinegar experiment is a captivating demonstration of a simple yet powerful chemical reaction. By understanding the science behind the fizz, following the step-by-step instructions, and taking necessary precautions, you can create a fun and educational experience for yourself or anyone you share it with. From simple volcano models to more complex investigations, the possibilities are endless. So, gather your materials, embrace the science, and prepare for an eruption of fun!
Frequently Asked Questions (FAQs)
1. Why does the reaction stop?
The reaction stops when either the baking soda or the vinegar runs out. Once one of the reactants is consumed, the reaction can no longer continue.
2. Can I use different types of vinegar?
Yes, you can use different types of vinegar, but white distilled vinegar is recommended because it’s clear and has a consistent acidity level. Apple cider vinegar can also work, but it may affect the color and clarity of the reaction.
3. What happens if I use too much baking soda or vinegar?
Using too much of either reactant won’t necessarily harm the experiment, but it might affect the intensity or duration of the reaction. It’s best to experiment with different ratios to see what works best.
4. Can I add other ingredients to the reaction?
Yes, you can experiment by adding things like dish soap (to create bubbles), food coloring (for visual appeal), or glitter (for extra sparkle). However, these additions are primarily for enhancing the aesthetics of the experiment and won’t significantly alter the chemical reaction itself.
5. Is the reaction dangerous?
The reaction is generally safe when conducted with adult supervision and basic safety precautions. Avoid getting vinegar in your eyes, and clean up any spills immediately.