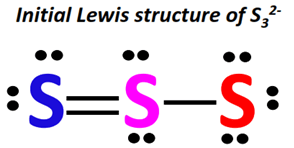S32 Lewis Structure Explained Beyond the Textbook: A Deep Dive
The Lewis structure, a cornerstone of introductory chemistry, allows us to visualize the bonding and electron distribution within molecules. While textbooks provide the basics, understanding the Lewis structure of S32 (a cyclic sulfur molecule) demands a deeper dive. This article goes beyond the standard explanation, exploring the nuances of this fascinating molecule, addressing common misconceptions, and equipping you with a comprehensive understanding.
Understanding the Basics: Sulfur’s Valence and Bonding
Before we construct the Lewis structure for S32, let’s revisit the fundamentals. Sulfur (S) belongs to Group 16 (also known as Group 6A) of the periodic table, placing it directly below oxygen. This position gives sulfur six valence electrons, meaning it has six electrons available for bonding.
- The Octet Rule: Sulfur, like oxygen, generally strives to achieve a stable octet configuration – eight electrons in its outermost shell – through bonding.
- Covalent Bonding: Sulfur atoms primarily form covalent bonds, sharing electrons to achieve this octet.
Constructing the S32 Lewis Structure: A Step-by-Step Approach
Now, let’s delve into constructing the Lewis structure for S32. This molecule exists as a ring of eight sulfur atoms, not a simple linear chain. Here’s how we arrive at the structure:
Total Valence Electrons: Calculate the total number of valence electrons. Each sulfur atom contributes six valence electrons, and with eight sulfur atoms, the total is 8 x 6 = 48 electrons.
Skeletal Structure: The S32 molecule forms a cyclic structure, a ring of eight sulfur atoms. Draw a ring of eight sulfur atoms, connecting each sulfur to its two adjacent neighbors with single bonds.
Electron Distribution and Completing Octets:
- Each single bond represents two shared electrons. Since we have eight single bonds, we’ve used 16 electrons (8 bonds x 2 electrons/bond).
- This leaves us with 32 electrons (48 total - 16 used in single bonds).
- Distribute the remaining electrons as lone pairs around the sulfur atoms to complete their octets. Each sulfur atom needs six more electrons (three lone pairs) to achieve a complete octet.
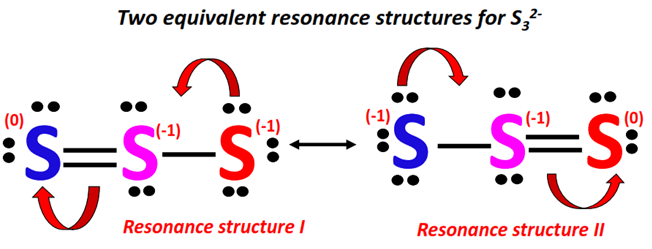
Formal Charge Calculation (Optional but Recommended): To assess the stability of the structure, calculate the formal charge on each sulfur atom.
- Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)
- In the ideal S32 Lewis structure, each sulfur atom will ideally have a formal charge of 0.
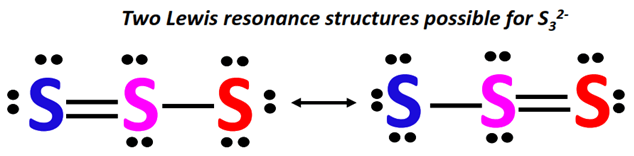
Resonance Structures: Due to the symmetry of the cyclic structure, the single bonds between sulfur atoms can be represented in multiple equivalent ways. This leads to the concept of resonance. The actual structure of S32 is a hybrid of these resonance forms, with the bonds being neither purely single nor purely double but somewhere in between.
Delving Deeper: Resonance and Bond Order in S32
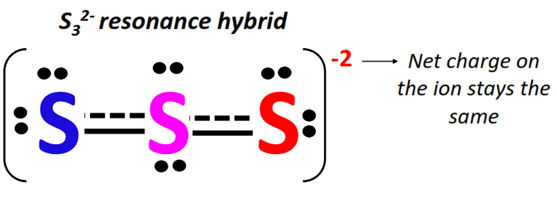
The S32 molecule exhibits resonance, a crucial aspect to understand. The bonds between the sulfur atoms are not fixed single bonds. Instead, the electrons are delocalized across the entire ring, leading to a bond order that is somewhere between a single and a double bond.
- Bond Order: The bond order in S32 is typically calculated to be around 1.33 (based on resonance), which is higher than a single bond and lower than a double bond.
- Stability and Reactivity: The delocalization of electrons through resonance contributes to the stability of the S32 molecule. This also influences its reactivity, making it less reactive than if it solely had single bonds.
Beyond the Diagram: Implications and Real-World Applications
Understanding the Lewis structure of S32 is crucial for several reasons:
- Predicting Properties: The Lewis structure helps predict the molecule’s shape, bond angles, and polarity, which in turn influences its physical and chemical properties.
- Understanding Reactivity: The resonance and bond order affect how S32 interacts with other molecules.
- Industrial Applications: Sulfur and its allotropes (like S32) find uses in various industries, including the production of sulfuric acid, rubber vulcanization, and the manufacturing of fertilizers.
Common Misconceptions about S32
- Thinking it’s a simple chain: The most common misconception is assuming S32 is a straight chain. Remember, it’s a cyclic structure.
- Ignoring Resonance: Failing to recognize the importance of resonance and the delocalization of electrons can lead to an inaccurate understanding of the bond order and stability.
- Focusing solely on Lewis Structure: While useful, the Lewis structure is a simplified representation. It doesn’t fully capture the 3D shape and electron distribution.
Frequently Asked Questions (FAQs)
Why does sulfur form a ring structure like S32? Sulfur atoms have a tendency to catenate, which means they can bond to each other to form chains or rings. This is due to the relatively strong S-S bond and the ability of sulfur to form multiple bonds. The ring structure of S32 is particularly stable due to the resonance stabilization.
Is S32 a polar or nonpolar molecule? S32 is a nonpolar molecule. The symmetrical ring structure and the even distribution of electron density ensure that there is no net dipole moment.
How does the Lewis structure of S32 relate to its allotropes? S32 is one of the allotropes of sulfur. Other allotropes, such as S6 and S8 (the most stable form), also exist. The Lewis structures of these allotropes differ in the number of sulfur atoms in the ring, impacting their properties.
Can we represent S32 using a VSEPR model? Yes, the Valence Shell Electron Pair Repulsion (VSEPR) theory can be applied. Each sulfur atom has two bonded atoms and two lone pairs, giving it a bent shape, however, as a ring structure, the overall molecular geometry is determined by the overall shape of the ring.
What is the significance of the bond order in S32? The bond order of approximately 1.33 in S32 indicates that the bonds are stronger than single bonds, which contributes to the molecule’s stability. It also affects the reactivity of the molecule.
Conclusion: Mastering the S32 Lewis Structure
Understanding the Lewis structure of S32 goes far beyond simply drawing dots and lines. It involves grasping the concepts of valence electrons, covalent bonding, resonance, and bond order. By going beyond the textbook and addressing the nuances of this fascinating molecule, you gain a deeper appreciation for the complexities of chemical bonding and the behavior of matter. The knowledge of S32, including its resonance structure and bond order, is key to understanding its properties and its relevance in the real world.