The Great Divide: Unpacking the “NO2 Polar or Nonpolar” Debate in Chemistry
The world of chemistry, with its intricate dance of electrons and molecules, often presents seemingly straightforward questions with surprisingly complex answers. One such question, frequently debated in classrooms and research labs alike, centers around the polarity of nitrogen dioxide (NO2). Is NO2 a polar molecule, or is it nonpolar? This seemingly simple query unlocks a deeper understanding of molecular structure, bond polarity, and the overall behavior of chemical compounds. This article dives deep into the heart of this debate, providing a comprehensive overview to help you understand the nuances and arrive at a well-informed conclusion.
Understanding the Basics: Polarity and Molecular Structure
Before we can determine the polarity of NO2, we need to establish some foundational concepts.
- Polarity: A molecule’s polarity refers to the uneven distribution of electron density within the molecule, resulting in partial positive (δ+) and partial negative (δ-) charges. This uneven distribution arises from differences in electronegativity between the atoms involved in the chemical bonds.
- Electronegativity: This is a measure of an atom’s ability to attract electrons in a chemical bond. The larger the electronegativity difference between two bonded atoms, the more polar the bond.
- Molecular Geometry: The three-dimensional arrangement of atoms within a molecule significantly influences its overall polarity. Even if individual bonds are polar, the molecule can be nonpolar if the polar bonds are arranged symmetrically, leading to a cancellation of dipole moments.
The Structure of Nitrogen Dioxide (NO2): A Key Player in the Debate
Nitrogen dioxide (NO2) is a reddish-brown gas with a characteristic pungent odor. Its structure plays a crucial role in determining its polarity.
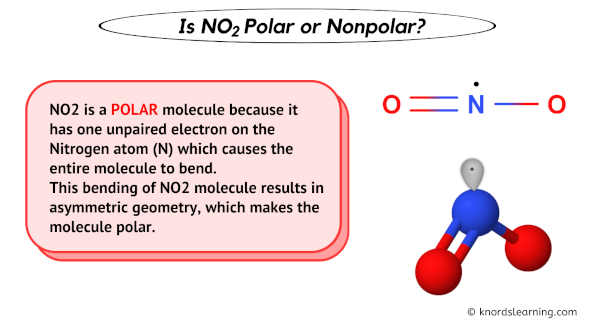
- Lewis Structure: The Lewis structure for NO2 shows a central nitrogen atom bonded to two oxygen atoms. It also reveals an unpaired electron on the nitrogen atom, making NO2 a radical. One oxygen atom forms a single bond, while the other forms a double bond (or, in reality, a delocalized bond through resonance).
- Molecular Geometry: Due to the presence of a lone electron and the bonds to the oxygen atoms, the molecular geometry is bent or angular. This bent shape is a critical factor in the polarity debate.
Arguments for NO2 Being Polar
Proponents of NO2 being a polar molecule often highlight the following points:
- Electronegativity Difference: Oxygen is significantly more electronegative than nitrogen. This difference in electronegativity leads to polar N-O bonds. The oxygen atoms attract the shared electrons more strongly, creating partial negative charges (δ-) on the oxygen atoms and a partial positive charge (δ+) on the nitrogen atom.
- Asymmetrical Structure: The bent shape of the molecule prevents the dipole moments of the two N-O bonds from perfectly cancelling each other out. The unequal distribution of electron density leads to a net dipole moment for the molecule.
- Experimental Evidence: Experimental measurements, such as dipole moment determination, consistently show that NO2 possesses a non-zero dipole moment, a characteristic feature of polar molecules.
Arguments for NO2 Being (Potentially) Less Polar or Exhibiting Complicated Behavior
While the points above strongly suggest polarity, some arguments complicate the picture:
- Resonance and Delocalization: The actual bonding in NO2 is best described by resonance, where the double bond is delocalized across both N-O bonds. This delocalization can make the molecule behave in ways that are more complicated than simple electronegativity differences might suggest.
- Unpaired Electron Influence: The unpaired electron on the nitrogen atom can influence the molecule’s interactions with other molecules, potentially leading to behaviors that aren’t purely dictated by its dipole moment. The radical nature of NO2 makes it more reactive, which can affect how it interacts with other molecules.
- Context Matters: The “polarity” of NO2 can be debated depending on the context, such as whether it is in the gas phase, dissolved in a solvent, or interacting with other molecules.
Reaching a Conclusion: A Complex Answer
The overwhelming evidence, including electronegativity differences, asymmetrical molecular geometry, and experimental data, supports the view that nitrogen dioxide (NO2) is a polar molecule. However, understanding the nuanced behavior of NO2 requires considering the influence of the unpaired electron, resonance, and the context in which it is observed. While the molecule possesses a net dipole moment and exhibits polar characteristics, its radical nature and delocalized bonding patterns add layers of complexity to its behavior.
FAQs: Frequently Asked Questions About NO2 Polarity
Here are a few common questions about NO2 polarity, answered:
Is NO2 a radical? Yes, NO2 is a radical due to the presence of an unpaired electron on the nitrogen atom. This unpaired electron significantly influences its chemical reactivity.
What is the effect of NO2’s polarity on its behavior? The polarity of NO2 allows it to interact with other polar molecules through dipole-dipole interactions. This can influence its solubility, boiling point, and reactivity.
How does the bent shape of NO2 contribute to its polarity? The bent shape prevents the individual bond dipoles from cancelling each other out, resulting in a net dipole moment and therefore, polarity. If the molecule were linear, the dipoles would cancel out, and the molecule would be nonpolar.
How does the resonance structure affect the polarity of NO2? While resonance doesn’t make NO2 nonpolar, it influences the distribution of electrons, making the bonds between the nitrogen and oxygen atoms more similar in character than a simple single and double bond model would suggest. This contributes to the molecule’s complexity and behavior.