Is Hydrogen Peroxide a Base? Science Explains All
Hydrogen peroxide, the ubiquitous solution found in medicine cabinets and cleaning supplies, is known for its bubbly reaction when encountering organic matter. But what exactly is hydrogen peroxide? Is it an acid, a base, or something else entirely? This article delves into the chemistry of hydrogen peroxide, providing a clear and comprehensive explanation of its properties and how it interacts with other substances. We’ll explore whether it fits the definition of a base, breaking down the science behind this versatile compound.
Understanding Acids and Bases: The Fundamentals
Before we can determine whether hydrogen peroxide is a base, we need to understand the fundamental concepts of acids and bases. Several theories define these crucial chemical categories:
- Arrhenius Definition:
- Acids: Substances that increase the concentration of hydrogen ions (H+) in a solution.
- Bases: Substances that increase the concentration of hydroxide ions (OH-) in a solution.
- Brønsted-Lowry Definition:
- Acids: Proton (H+) donors.
- Bases: Proton (H+) acceptors.
- Lewis Definition:
- Acids: Electron pair acceptors.
- Bases: Electron pair donors.
These definitions provide different perspectives on how acids and bases behave, but they all highlight the fundamental differences in their chemical properties.
Delving into Hydrogen Peroxide (H₂O₂)
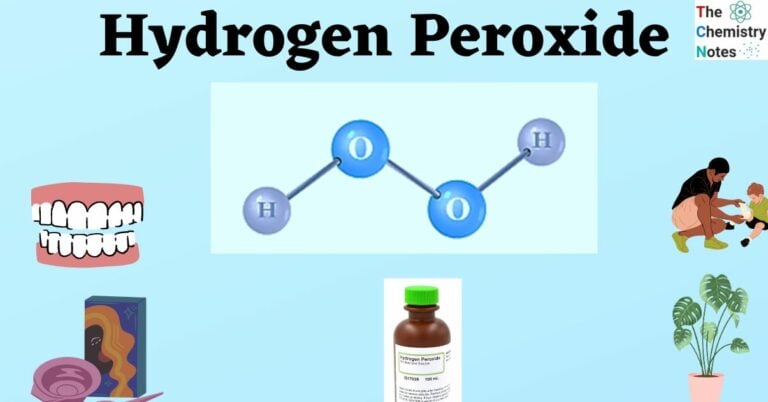
Hydrogen peroxide (H₂O₂) is a chemical compound composed of two hydrogen atoms and two oxygen atoms. Its structure, unlike water (H₂O), features an oxygen-oxygen single bond, making it inherently unstable and prone to decomposition. This instability is what gives hydrogen peroxide its oxidizing power and its ability to act as a disinfectant and bleaching agent.
- Key Properties of Hydrogen Peroxide:
- Chemical Formula: H₂O₂
- Appearance: Typically a colorless liquid
- Odor: Faint, characteristic odor
- Decomposition: Decomposes into water (H₂O) and oxygen gas (O₂)
- Oxidizing Agent: Readily accepts electrons from other substances.
- Weak Acid: Can donate a proton (H+), behaving as a weak acid in some reactions.
Hydrogen Peroxide and the Definition of a Base: The Answer
So, is hydrogen peroxide a base? The answer is no, it is not generally considered a base.
While hydrogen peroxide can sometimes act as a base in specific reactions, it doesn’t fit the primary characteristics of a base according to the most common definitions:
- Arrhenius: It doesn’t directly release hydroxide ions (OH-) in solution.
- Brønsted-Lowry: It can accept a proton (H+), but this is less common than its acidic behavior.
- Lewis: It can act as an electron pair donor in some specific reactions, making it sometimes act as a base.
Hydrogen peroxide is more accurately described as a weak acid due to its ability to donate a proton, albeit weakly. Its primary chemical behavior revolves around its oxidizing properties.
The Acidic Nature of Hydrogen Peroxide
Although hydrogen peroxide isn’t a strong acid, it can still donate a proton (H+) in certain circumstances, making it a weak acid. This means it can slightly increase the concentration of hydrogen ions (H+) in a solution, albeit to a lesser extent than stronger acids.
Hydrogen Peroxide Applications and Reactions
Hydrogen peroxide’s unique properties make it a valuable compound in various applications:
- Disinfectant and Antiseptic: Kills bacteria and other microorganisms.
- Bleaching Agent: Used to whiten hair, teeth, and fabrics.
- Rocket Propellant: Used in some rocket engines due to its decomposition into oxygen gas.
- Industrial Applications: Used in various industrial processes, such as wastewater treatment.
The reactions hydrogen peroxide undergoes often involve its oxidizing power, where it accepts electrons from other substances, rather than its basic properties.
Conclusion: The Chemistry of Hydrogen Peroxide Explained
In conclusion, while hydrogen peroxide might show some basic behavior in some specific reactions, it is primarily a weak acid and a powerful oxidizing agent. It does not fit the general definition of a base, as it doesn’t typically release hydroxide ions or readily accept protons. Understanding the chemical properties of hydrogen peroxide allows us to appreciate its diverse applications and its role in various chemical processes.
Frequently Asked Questions (FAQs)
1. Can hydrogen peroxide neutralize acids?
Yes, hydrogen peroxide can react with acids, but it won’t neutralize them in the traditional sense of a base neutralizing an acid (producing salt and water). The reaction usually involves the oxidation of the acid or a component of the acid.
2. What is the pH of hydrogen peroxide?
The pH of hydrogen peroxide solutions depends on the concentration. Typically, a 3% solution has a pH of around 4-5, making it slightly acidic.
3. Is hydrogen peroxide safe to use?
Hydrogen peroxide is generally safe to use in diluted forms (like 3% solutions). However, it can cause irritation if it comes into contact with skin or eyes. Always follow the manufacturer’s instructions and handle it with care. Concentrated hydrogen peroxide is extremely dangerous and can cause severe burns.
4. Does hydrogen peroxide have a specific taste?
Yes, hydrogen peroxide has a slightly bitter taste. However, it is not recommended to ingest it.